WE'RE HIRING
We Are Looking for Passionate People
People are the energy behind our organization, and we’re committed to fostering their success in and out of the office. Visit our job opening page to view careers at ANI.
Our Mission is...

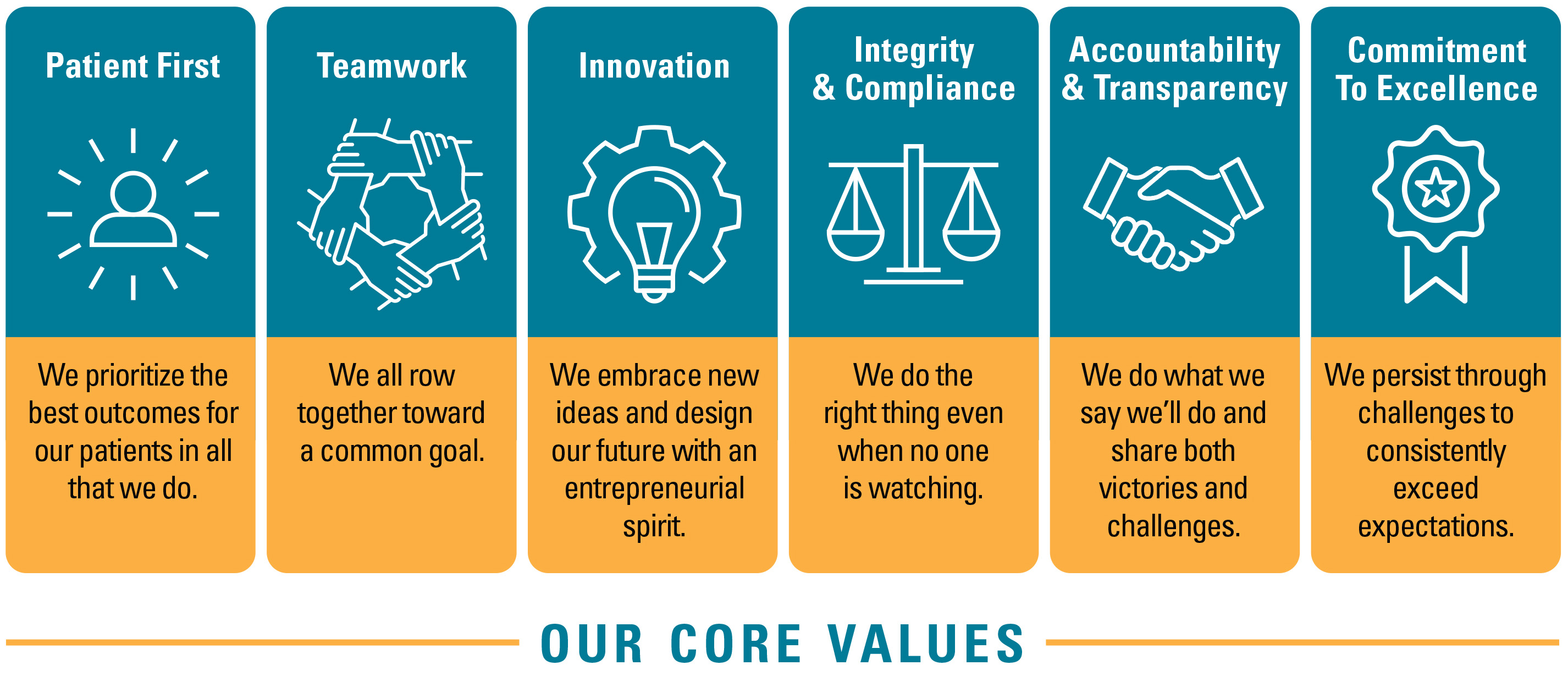
Our Values
We've become aware of individuals falsely claiming to represent ANI Pharmaceuticals in recruitment communications. To protect yourself, please note:
- All official recruiting emails come from @anipharmaceuticals.com domains only
- Our recruiters will always be connected to our company page on LinkedIn
- We never request payment, personal financial information, or sensitive personal details during the recruitment process
- You can verify job postings on our official job postings page at jobs.anipharmaceuticals.com
- Always apply through our website – click the “apply now” button for the job you’re interested in


